Henry Shaw, UC Master Food Preserver Online Program Volunteer
“Water activity” or “the activity of water” (typically abbreviated as “aw”) is a critical parameter in food science that influences microbial growth and chemical reactions (including enzymatic processes) in food products. Unlike moisture content, which measures the total amount of water in a product, water activity quantifies the availability of water for microbial and chemical processes.
Formally, the activity of water in a food sample is defined as the ratio of the vapor pressure of water in that sample to the vapor pressure of distilled water under identical conditions (U.S. Food & Drug Administration, 1984). It can take on values ranging from 0 (completely dry) to 1.0 (pure water). This formal definition can seem like a lot of hard-to-understand scientific gibberish, but there’s a concept that most of us are familiar with that can help us understand the definition. The water activity of a food sample is simply the relative humidity (RH) in the air in a sealed jar that contains the food in question after that food has had time to equilibrate with the air. Relative humidity is usually reported as a percentage, but if we express the percentage as a decimal fraction (i.e., divide the percent relative humidity by 100) we get the water activity: aw = RHjar/100.
At 100% relative humidity (aw = 1) it’s raining; the air contains as much water vapor as it can hold at that temperature. At lower atmospheric relative humidities, “wet” things (i.e., those with an aw greater than RH/100) will dry out. If we seal a jar with a food product and let it equilibrate, the food will determine the relative humidity in the air in that jar by either evaporating water from the product or absorbing water vapor from the original air in the headspace. The final relative humidity in the headspace, divided by 100, is the activity of water in that product.
Microbial growth is highly dependent on water activity and the goal in food preservation is to lower the aw to a value at which pathogens and food-spoilage organisms cannot thrive. Most bacteria require a water activity above 0.91 to grow. Most yeasts can only survive at water activities above 0.88, and molds can survive at even lower water activities, with a limit of about aw ~ 0.65. By reducing the water activity in a product, we can inhibit the growth of these spoilage organisms and pathogens, thereby extending the product’s shelf life and enhancing food safety. For reference, the horizontal dashed lines in Figure 1 show the water activity levels below which various types of food spoilage organisms can no longer grow.
In addition to affecting microbiological activity, the activity of water influences chemical reactions such as lipid (fat) oxidation and Maillard browning. These reactions can lead to undesirable changes in flavor, color, and nutritional quality. By lowering water activity, we can slow these reactions and maintain product quality and prolong storage life.
Food preservation methods like dehydration, freezing, and the addition of humectants (things that “bind” with water and reduce its availability, e.g., salt or sugar) are commonly used to reduce water activity. Dehydration is an effective means for reducing the activity of water. This process physically removes water to lower the aw. Dried fruits, jerky, and powdered milk are preserved in this way and typically have water activities less than 0.75, which is below the threshold needed for most microbial growth (see Fig. 1). Note that when we “condition” dehydrated foods prior to long-term storage (i.e., let them sit in a closed jar for a week or so, shaking the jar daily), what we are doing is letting the dried pieces of food equilibrate so that the aw becomes the same in each piece. If we have different types of food in that jar (e.g., a mixture of dehydrated vegetables for use in later soup making), the activity of water in each piece will be the same after conditioning, but the water content of each type of vegetable may well be different due to the differing chemical compositions of the different vegetables.
Freezing immobilizes water molecules (and slows both the metabolic activity of bacteria and the rate of chemical reactions responsible for food degradation). Humectants bind free water to reduce its chemical availability. For instance, as shown in Figure 1, a 13 wt. % solution of salt (NaCl) has a water activity of ~0.91, which is low enough to suppress the growth of most “ordinary” bacteria. In contrast, one needs a 55 wt. % solution of sugar to reach the same aw. On a weight basis, therefore, salt is much more effective at reducing aw in a water solution than sugar.
All these techniques lower the activity of water in preserved foods to prevent spoilage and ensure that food remains safe for consumption after storage.
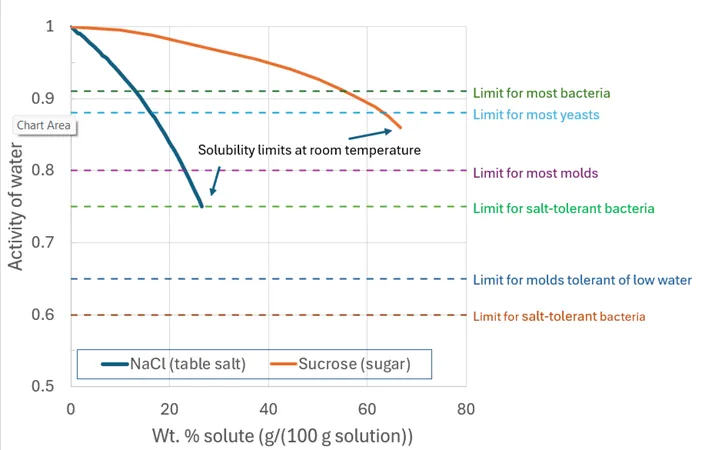
Figure 1. The activity of water in salt and sugar solutions as a function of solute concentration. The two curves show how increasing salt or sugar concentrations lower the activity of water in the solution. Both curves stop at the solubility limit of the solute at room temperature. Note that the weight percent of the solution on the horizontal axis is the mass (weight) of the sugar or salt divided by mass of the water plus the mass of the sugar or salt in the solution. Dashed horizontal lines indicate the growth limits of various food-degradation organisms; at an aw lower than a given line, an organism will not grow. (Data from Gekas et al., 1998; FDA, 2012; Bressan and Mathlouthi, 1993; Fundamentals of Consumer Food Safety and Preservation, 2018)
References:
Bressan, C. and M. Mathlouthi, 1993, Thermodynamic activity of water and sucrose and the stability of crystalline sugar. AVH Association 1st Symposium, Reims, France.
Food and Drug Administration, 2012, Bad Bug Book, Foodborne Pathogenic Microorganisms and Natural Toxins. Second Edition. Appendix 3. Factors that Affect Microbial Growth in Food, p 261.
Gekas, V., C. Gonazlez, A Sereno, A Chiralt, and P. Foto, 1998, Mass transfer properties of osmotic solutions. 1 Water activity and osmotic pressures. Intl. J. of Food Properties, I(2) 95-112.
U.S. Food and Drug Administration, 1984, Water Activity (aw) in Foods. Inspection Technical Guides No. 39, https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-technical-guides/water-activity-aw-foods.
Washington State University and University of California, 2018, Fundamentals of Consumer Food Safety and Preservation, Master Handbook, p 1-10.
