Regular Haven visitors notice that we frequently change our planting. It's one of the joys of gardening -- there are always new plants to try and experiment with. At the Haven, research on new plants and methods for bee gardens is essential to our educational mission.
Previous posts have reported on our work with bee plant preferences, with an emphasis on low-water plants. Our colleagues at UC Cooperative Extension in Southern California have done similar work for that part of the state. Previous blog posts have covered research with mulch, plant color, and water; all are important components of a healthy bee garden.
But all this is for naught if it isn't put into practice. So our current work is to create tools for the California horticulture industry to educate employees and customers about bee garden best practices based on this research. The first step in this work is development of an efficient sampling method so growers, landscapers, and public gardens can easily assess the bee-attractiveness of new plants as they come on the market. This will allow these plants to be marketed correctly, and will also help growers to target bee-friendly pest management.
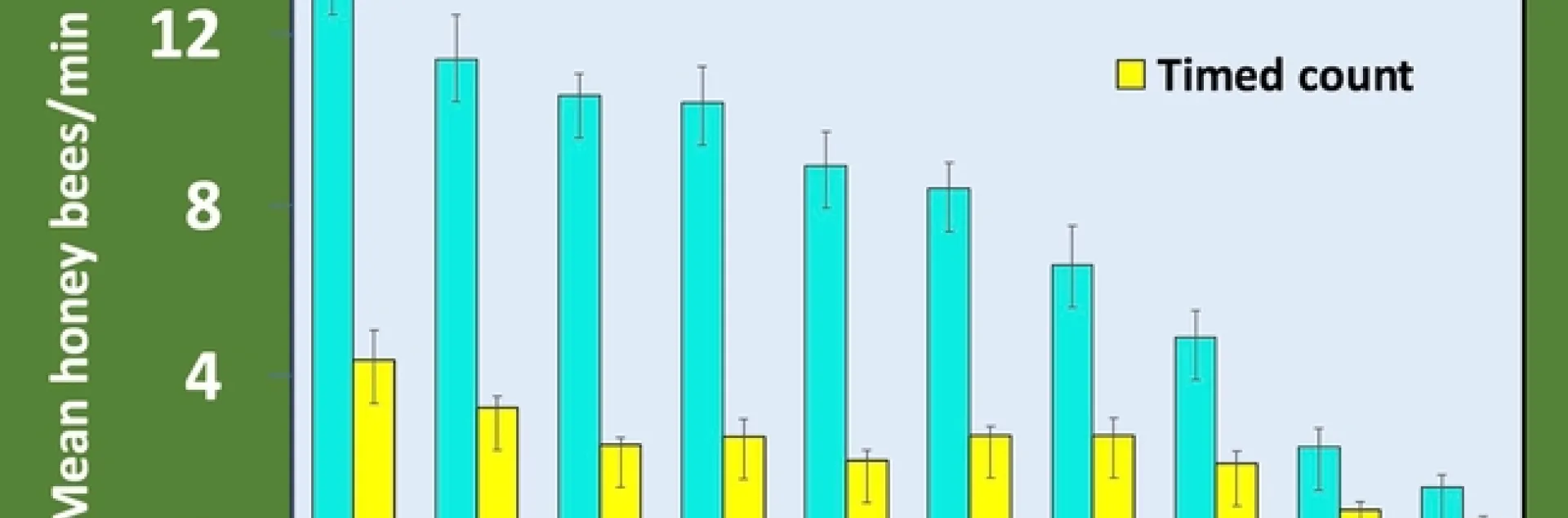
In our attractiveness studies the Southern CA team used timed counts, while the Northern CA team used a snapshot count method (1). This technique consists of 20 second quick counts of every plant that is repeated three times in succession rather than a single 3-minute timed count. The snapshot count is faster to complete and more readily worked into the day of an otherwise busy nursery employee.
The purpose of this study was to calculate the relative net precision (RNP) of each bee counting method at a wholesale nursery (Fallbrook, CA) and a public garden site (Encinitas, CA) in San Diego County. RNP is calculated as shown below and is a way to assess sampling efficiency by balancing precision and sampling cost (2).
RNP = [1/(cost x Rv)] x 100, where Rv = (SE/mean) x 100
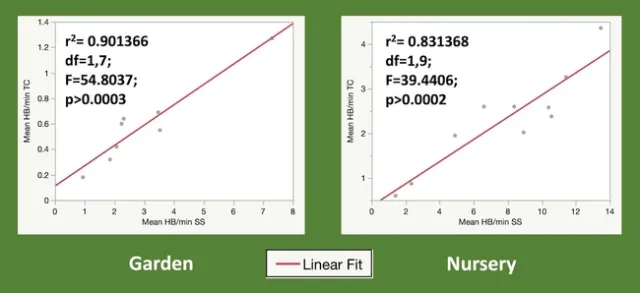
Plants in full bloom were sampled weekly for at least 4 weeks using both methods. Bees were counted as honey bees or other bees as this distinction is easy for an untrained observer; only honey bee data is reported here. At both locations, we saw a larger absolute number of bees with the snapshot method, but the trend of most attractive to least attractive was the same for both methods (Figures 1 and 2). We are most interested in this trend rather than the absolute number, which is expected to vary between locations. Additionally, regression analysis shows that the two counting methods are strongly correlated (Figure 3).
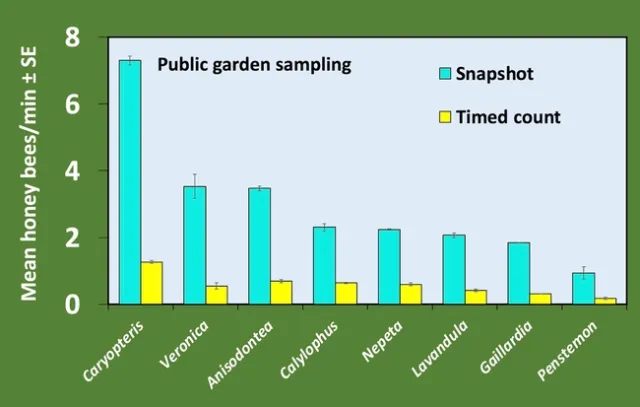
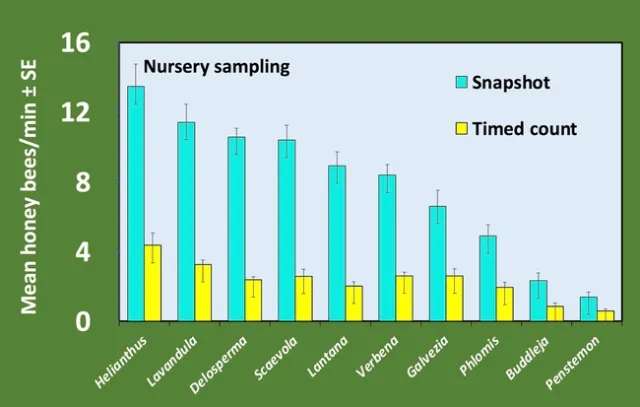
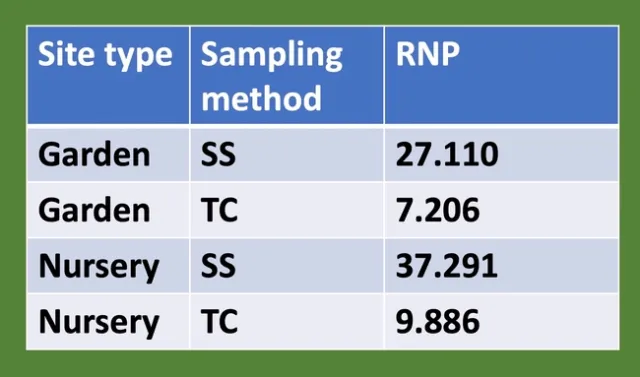
Finally, we saw differences between the RNP values calculated for the two sampling methods, with higher RNP for the snapshot method at both locations (Table 1). Higher RNP means greater sampling efficiency (2).
A second year of study will begin in April at additional sites to confirm these findings. We look forward to providing the California green industry with a useful tool for supporting pollinator gardens.
References
1. Garbuzov and Ratnieks. 2014. Functional Ecology 28: 364-374.
2. Buntin pp 99-115 in Handbook of Sampling Methods for Arthropods in Agriculture. CRC Press, Boca Raton, FL. 1994.